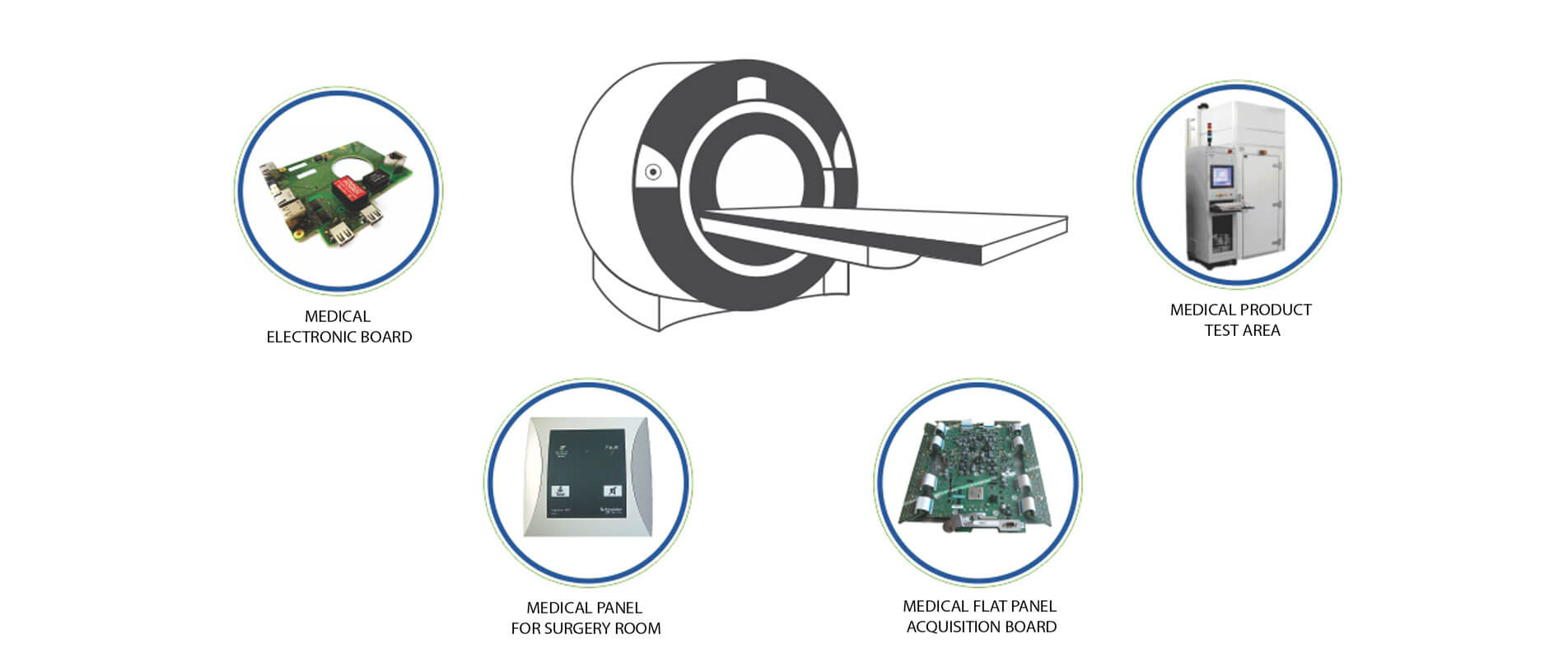
Medical
The field of healthcare is rapidly adopting new technologies to augment the quality of treatment and create efficiencies for healthcare providers. At Centum, our engineering and manufacturing teams have worked on a variety of medical devices and equipment that include digital radiography systems, automated pump for drug injection, ultrasound equipment, patient monitoring devices, customized room controls for operation theaters among others.
Our engineering and manufacturing teams have developed a good understanding of the applications and have the competencies to design and manufacture product with a high level of reliability. We have experience working with the design and manufacturing standards listed in the table below.
| ISO 13485 | Quality systems – Medical devices – Particular requirements for the application of ISO 9001. |
| IEC CISPR11 / EN55011 | Industrial, scientific & medical radiofrequency equipment – electromagnetic disturbance characteristics – limits & methods of measurements. |
| IEC 62304 / prEN 624 | Medical device software – Software life-cycle processes. |
| IEC / EN 60601-1-4 | Medical Equipment – Part1: General requirements for safety collateral standard 4: Programmable electrical medical systems. |
| IEC 60068 series | Environment Tests. |
| ISO 14971 | Medical Devices – Application of risk management to medical devices. |
| IEC 60825-1 | Safety of laser products – Part 1: Equipment classification and requirements. |
| IEC60825-2 | Safety of laser products – Part 2: Safety of optical fiber communication systems (OFCS). |
| IEC 60664 | Insulation requirements (Over Voltage Category III, pollution degree 2). |
| IEC 61000-4-5 level 4 | Electromagnetic compatibility (EMC) – Part 4-5: Testing and measurement techniques – Surge immunity test. |
| IEC 60364-7-710 | Requirements for special installations or locations Medical locations. |
| IEC 61326-2-4 | Electrical equipment for measurement, control and laboratory use. EMC requirements. Particular requirements. Test configurations, operational conditions and performance criteria for insulation monitoring devices. |
| IEC 60601-1 | Medical Equipment – Part 1: general requirements for safety |
| IEC 61557-8 | Electrical safety in low voltage distribution systems up to 1 000 V AC and 1 500 V DC – Equipment for testing, measuring or monitoring of protective measures – Part 8: Insulation monitoring devices for IT systems. |
PRODUCT REFERENCES